General Formula of Alkane
General formula of alkene is Cn H2n and it is different form alkane general formula which is CnH2n2 where n is integer. The general formula of alkanes is C n H 2n2 indicating that for every n carbon atom alkanes have 2 n 2 hydrogen atoms.

Naming Isomers 3 Structural Formula Molecular Study Tips
Members of a Homologous Series.
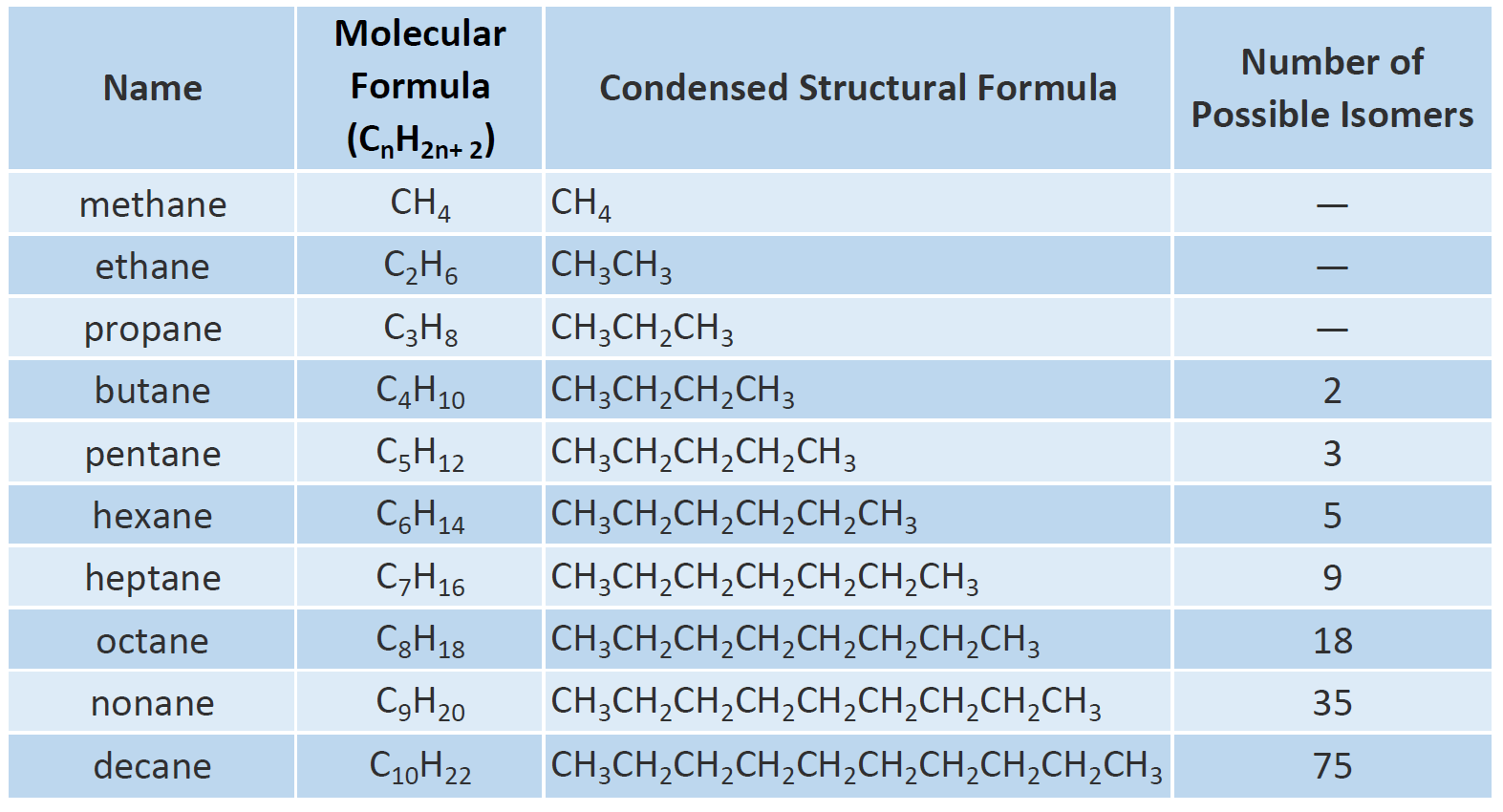
. The alkanes range in complexity from the simplest case of methane CH4. The general formula for the cycloalkane is C n H 2 n. Alkanes are represented by the general formula C n H 2n2.
The alkanes are a family of simple hydrocarbons with general formula CnH2n2 where n 1 2 3. The general formula for the linear and branched alkanes is C n H 2 n 2. Likewise why is the.
The question tells us that butane has four carbon atoms and so here n 4. C n H 2n2 2. Its molecules contain 10 carbon atoms.
Answer 1 of 30. Alkanes Formula and its Condensed. BY reason of their formula alkanes are said to.
In other words an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carboncarbon bonds are single. Each carbon atom is bonded to four. N2 for an alkene.
The general formula for the alkenes is C n H 2n where n is the number of carbon atoms in the molecule. Alkanes are saturated hydrocarbons. In organic chemistry an alkyne is an unsaturated hydrocarbon containing at least one carbon carbon triple bond.
N1 for an alkane. Alkanes have the general chemical formula CnH2n2. The general formula of alkane is- 1.
C n H 3n3 4. To use the general formula replace n in the general formula with the number. We can see from the formula that alkanes h 2n 2.
C3H8 propane 2 3 6 6 2. That is the number of hydrogen atoms the alkane will have. Multiply the number of carbons by two then add two to that result.
General formula for alkenes. C n H 2n. General formula for alkanes.
IUPAC System of Alkenes. A fully saturated hydrocarbon an alkane has general formula C_nH_2n2. The examples of the Alkane are CH 4 Methane and C 2 H 5.
Ethers R-O-R are compounds formed by replacing hydrogen atoms of an alcohol R-OH compound or a phenol C6H5OH by an aryl acyl group functional group after removing. General Formula of Alkanes Video Lecture from Alkanes Chapter of Chemistry Class 11 for HSC IIT JEE CBSE NEETWatch Previous Videos of Chapter Alkanes-1. The Lewis structure of alkanes can be simplified using the.
Alkanes are organic compounds that consist of single-bonded carbon and hydrogen atoms. Note that this is the same as the alkane formula except that we subtract two hydrogen atoms to allow for the double bond. N4 for an alcohol.
Each succeeding formula incorporates one carbon atom and two hydrogen atoms more than the previous formula. What is alkanes write the general formula and properties of alkanes. Each homologous series has its own general formula.
What is the general formula of alkane and give some examples 1 See answer Advertisement Advertisement dev0to100777 dev0to100777 Explanation. Share It On Facebook Twitter Email. Decene is an alkene.
C n H 2n2 3. Alkenes bear the general formula CnH2n. 1 The simplest acyclic alkynes with only one triple bond and no.
C n H 2n2. In organic chemistry an alkane or paraffin a historical trivial name that also has other meanings is an acyclic saturated hydrocarbon.

Alkanes Vs Alkenes Vs Alkynes Chemistry Lessons Chemistry Worksheets Chemistry Basics

Ch105 Chapter 7 Alkanes And Halogenated Hydrocarbons Chemistry

Class 10 General Formula Of Alkane Alkene Alkyne Tx Academy Youtube
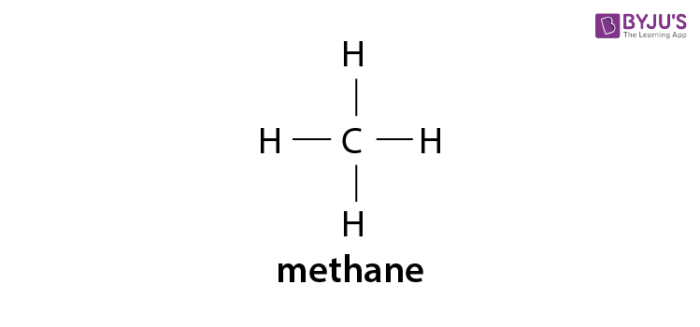
Alkanes Formula Definition Structure Properties List Of Alkanes Videos Examples And Faqs Of Alkanes
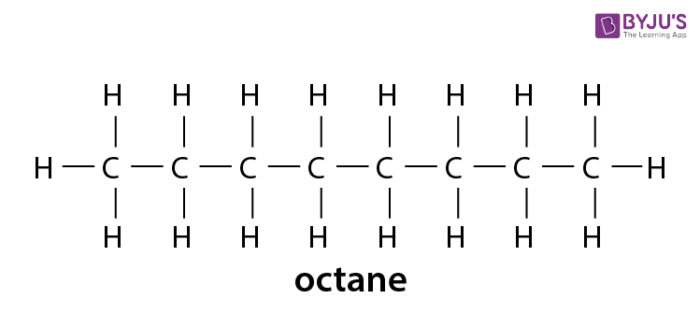
Alkanes Formula Definition Structure Properties List Of Alkanes Videos Examples And Faqs Of Alkanes
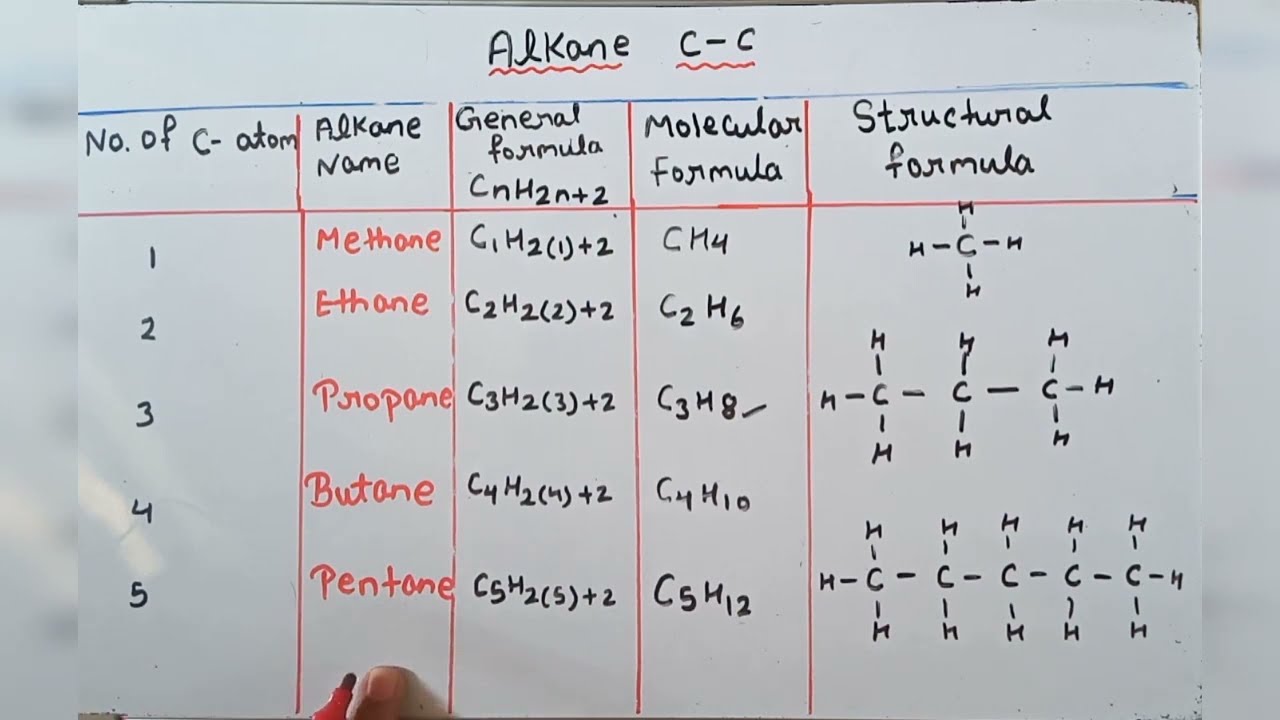
Alkane Molecular Structural General Formula Youtube
0 Response to "General Formula of Alkane"
Post a Comment